-
宽叶金粟兰(Chloranthus henryi)是金粟兰科(Chloranthaceae)金粟兰属(Chloranthus)多年生草本植物,又名大叶及已、红四块瓦、四叶对、四大天王和四大金刚等,主要分布于我国贵州、四川、广西、安徽等地。宽叶金粟兰的根、根状茎和全草均可做药用,在《全国中草药汇编》《中药大辞典》《中华本草》中均有收载,其性辛、苦、温、有毒,具有散寒止咳,活血止痛,散瘀解毒等功效。民间多用于风寒咳嗽、风湿骨痛、四肢麻木、月经不调、闭经和小儿惊风等。外用治跌打损伤,淤血肿痛,毒蛇咬伤(曹聪梅等,2008)。现代药理研究表明,金粟兰属植物含有倍半萜类、倍半萜二聚体类、苯丙素类和酰胺类等化学成分,具有抗菌、抗炎、抗肿瘤、抗HIV-1、增强免疫、神经保护、利胆和收缩子宫等生物活性(罗杠等,2014;杨秀伟,2017)。
-
目前,关于宽叶金粟兰倍半萜二聚体类单体化合物的研究报道较少,仅张珊珊等(2017)从宽叶金粟兰中分离得到5个倍半萜二聚体类化合物。本文进一步对宽叶金粟兰的化学成分及细胞毒活性进行研究,以期发现结构新颖的倍半萜二聚体类化合物,有助于该植物的开发及利用。
-
本研究以宽叶金粟兰为研究对象,依托现代先进的仪器设备,采用硅胶柱层析、反相柱色谱、Sephadex LH-20凝胶和半制备高效液相等色谱技术对宽叶金粟兰95%乙醇提取物的乙酸乙酯部位进行分离纯化,并采用MTT法评价所得化合物的细胞毒活性,通过理化性质与波谱数据并结合参考文献鉴定所得化合物的结构及细胞毒活性测试结果,拟探讨以下问题:(1)宽叶金粟兰95%乙醇提取物的乙酸乙酯部位的化学成分;(2)所分离得到的化合物的细胞毒活性。
-
1 实验材料
-
1.1 药材来源
-
实验所用宽叶金粟兰药材(100.0 kg)采集于贵州安龙,经中国科学院昆明植物研究所刘海洋研究员鉴定为宽叶金粟兰(Chloranthus henryi)药材全草,标本(Assrmyy20180819-2)存放于安顺市人民医院药剂科药学实验室。
-
1.2 实验仪器及试剂
-
INOVA-600 MHz超导核磁共振波谱仪(美国瓦里安公司),TMS为内标;Waters 1525 EF高效液相色谱仪(美国 Waters 公司),Waters 2998检测器,Waters sunfire色谱柱为 C18(10 mm × 250 mm,4.6 mm × 150 mm);JASCO P-1020旋光仪(日本 JASCO 公司);Shimadzu UV-2401A紫外光谱仪(日本 Shimadzu 公司);Bruker Tensor-27红外光谱仪(德国 Bruker 公司);Bruker HCT/Esquire和Waters Autospec Premier P776质谱仪(德国 Bruker 公司,美国 Waters 公司);Sephadex LH-20(美国 GE Healthcare Bio-sicence AB 公司);MCI GEL CHP20(日本三菱化学公司);柱层析硅胶(40~80目和300~400目,青岛海洋化工厂);硅胶GF254薄层板(50 mm × 100 mm,0.20~0.25 mm,青岛海洋化工厂);分析纯三氯甲烷、丙酮(重庆川东化工集团有限公司);其余试剂均从工业级蒸馏中获得;10%硫酸乙醇显色剂。
-
2 实验方法
-
2.1 提取与分离
-
将干燥的宽叶金粟兰全草(100.0 kg)粉碎后,用95%乙醇在90℃下加热回流提取3次,每次3.5 h,将提取液合并,减压浓缩回收乙醇,得到宽叶金粟兰浓缩液。取少量浸膏留样,其余浓缩液经乙酸乙酯萃取,得到乙酸乙酯萃取物7.4 kg。将萃取物用溶剂溶解,利用粗硅胶(40~80目)拌样,待样品干燥后,采用经硅胶(300~400目)柱色谱(石油醚-丙酮50∶1→1∶1,V/V)进行梯度洗脱,洗脱部分用TLC薄层板展开,在紫外分析仪下观察TLC薄层板荧光显色情况,并用10%硫酸乙醇溶液显色,合并相似部分,最后用甲醇处理硅胶柱,得到18个馏分(Fr.1-Fr.18)。
-
Fr.13经D-101大孔树脂柱层析(EtOH∶H2O 20∶80 → 95∶5,V/V)进行梯度洗脱,得到5个组分(Fr.13.1-Fr.13.5)。Fr.13.2经硅胶柱色谱(石油醚-丙酮 10∶1→1∶1,V/V)进行梯度洗脱,并用葡聚糖凝胶Sephadex LH-20柱色谱(MeOH)和半制备液相色谱(CH3CN∶H2O 45∶55,V/V)进一步纯化,分离得到化合物4(tR= 12.0 min,13.2 mg)。Fr.13.3经硅胶柱色谱(石油醚-丙酮10∶1→1∶1,V/V)进行梯度洗脱,得到4个组分(Fr.13.3.1-Fr.13.3.4)。Fr.13.3.2经葡聚糖凝胶Sephadex LH-20柱色谱(CHCl3∶MeOH 1∶1,V/V)洗脱,分离得到化合物1 (22.2 mg)。Fr.13.3.4经葡聚糖凝胶Sephadex LH-20柱色谱(MeOH)洗脱,得到3个组分(Fr.13.3.4.1-Fr.13.3.4.3)。Fr.13.3.4.3经硅胶柱色谱(石油醚-丙酮10∶1→1∶1,V/V)进行梯度洗脱,分离得到化合物5(10.2 mg)。
-
Fr.14经硅胶柱色谱(石油醚-乙酸乙酯2∶1→1∶1,V/V)进行梯度洗脱,分离得到5个组分(Fr.14.1-Fr.14.5)。Fr.14.5经葡聚糖凝胶Sephadex LH-20柱色谱(CHCl3∶MeOH 1∶1,V/V)洗脱,得到3个组分(Fr.14.5.1-Fr.14.5.3)。Fr.14.5.3经硅胶柱色谱(石油醚-丙酮5∶1→1∶1,V/V)进行梯度洗脱,分离得到5个组分(Fr.14.5.3.1-Fr.14.5.3.5)。Fr.14.5.3.4经硅胶柱色谱(石油醚-丙酮5∶1→1∶1,V/V)进行梯度洗脱,分离得到11个组分(Fr.14.5.3.4.1-Fr.14.5.3.4.11)。Fr.14.5.3.4.10经硅胶柱色谱(石油醚-丙酮4∶1→1∶1,V/V)进行梯度洗脱,分离得到化合物12(31.7 mg)。Fr.14.5.3.4.5经葡聚糖凝胶Sephadex LH-20柱色谱(MeOH)洗脱,得到4个组分(Fr.14.5.3.4.5.1-Fr.14.5.3.4.5.4)。Fr.14.5.3.4.5.2经反相硅胶(MeOH∶H2O 40∶60 → 90∶10,V/V)进行梯度洗脱,得到5个组分(Fr.14.5.3.4.5.2.1-Fr.14.5.3.4.5.2.5)。Fr.14.5.3.4.5.2.1经硅胶柱色谱(氯仿-甲醇150∶1→50∶1,V/V)进行梯度洗脱并重结晶得到化合物11(41.9 mg)。Fr.14.5.3.4.5.2.5经硅胶柱色谱(氯仿-甲醇 100∶1→50∶1,V/V)进行梯度洗脱和半制备液相色谱(CH3CN∶H2O 35∶65,V/V)进一步纯化,分离得到化合物6(tR= 18.5 min,18.2 mg)。
-
Fr.15经葡聚糖凝胶Sephadex LH-20柱色谱(CHCl3∶MeOH 1∶1,V/V)洗脱,得到4个组分(Fr.15.1-Fr.15.4)。Fr.15.3经硅胶柱色谱(石油醚-丙酮5∶1→1∶1,V/V)进行梯度洗脱,得到5个组分(Fr.15.3.1-Fr.15.3.5)。Fr.15.3.1经MCI(MeOH∶H2O 40∶60 → 90∶10,V/V)进行梯度洗脱,得到7个组分(Fr.15.3.1.1-Fr.15.3.1.7)。Fr.15.3.1.6经D-101大孔树脂柱层析(EtOH∶H2O 20∶80 → 95∶5,V/V)进行梯度洗脱,分离得到化合物7(1.70 g)并得到9个组分(Fr.15.3.1.6.1-Fr.15.3.1.6.9)。Fr.15.3.1.6.7经硅胶柱色谱(石油醚-乙酸乙酯5∶1→1∶1,V/V)进行梯度洗脱,得到5个组分(Fr.15.3.1.6.7.1-Fr.15.3.1.6.7.5)。Fr.15.3.1.6.7.4经硅胶柱色谱(二氯甲烷-甲醇100∶1→1∶1,V/V)进行梯度洗脱,并用半制备液相色谱(CH3CN∶H2O 45∶55,V/V)进一步纯化,分离得到化合物2(tR= 31.0 min,12.3 mg)和化合物3(tR= 14.0 min,9.6 mg)。Fr.15.3.5经葡聚糖凝胶Sephadex LH-20柱色谱(MeOH)洗脱,得到6个组分(Fr.15.3.5.1-Fr.15.3.5.6)。Fr.15.3.5.2经反相硅胶(MeOH∶H2O 40∶60 → 90∶10,V/V)进行梯度洗脱,得到8个组分(Fr.15.3.5.2.1-Fr.15.3.5.2.8)。Fr.15.3.5.2.3经硅胶柱色谱(石油醚-丙酮10∶1→1∶1,V/V)进行梯度洗脱并重结晶得到化合物9。Fr.15.3.5.3经硅胶柱色谱(石油醚-丙酮10∶1→1∶1,V/V)进行梯度洗脱,得到5个组分(Fr.15.3.5.3.1-Fr.15.3.5.3.5)。Fr.15.3.5.3.2经硅胶柱色谱(二氯甲烷-甲醇100∶1→1∶1,V/V)进行梯度洗脱,并用半制备液相色谱(CH3CN∶H2O 60∶40,V/V)进一步纯化,分离得到化合物10(tR= 9.0 min,8.3 mg)和化合物8(tR= 16.0 min,13.1 mg)。
-
2.2 细胞毒活性实验
-
采用MTT法检测宽叶金粟兰的化学成分对人宫颈癌细胞HeLa、人乳腺癌细胞MCF-7、人前列腺癌细胞DU-145、人肺癌细胞A549和人脑胶质瘤细胞T98G细胞毒性作用。称取一定量的化合物分别用DMSO溶解后配成30 mmol·L-1的储备液,使用之前稀释至20 μmol·L-1,在5% CO2、37℃孵育72 h后进行初筛活性,得到效果显著的化合物。将肿瘤细胞分别配制成细胞悬液接种于96孔板上(每孔100 μL),设置阳性对照组(紫杉醇)和浓度梯度给药组,将接种好的细胞培养板放入培养箱中培养24 h后分别加入0.625、1.25、2.5、5、10 μmol·L-1的含药样品溶液各100 μL,在5% CO2、37℃孵育72 h后,每孔加入10 μL配制好的 MTT溶液(5 mg·mL-1,即0.5% MTT)继续培养4 h,孵育后使用酶标仪测量OD值,并计算IC50。
-
3 结构鉴定
-
化合物1 白色粉末; -5.22(c 0.1,MeOH),UV光谱(MeOH)显示在235 nm处有最大吸收;IR谱图显示存在羰基(1 736和1 645 cm-1)、氨基(3 256和2 925 cm-1)和苯环(700 cm-1)等特征吸收峰信号。高分辨分子离子峰HR-ESI-MS为m/z 591.248 9 [M + H] +(计算值:C36H34N2O6H,591.248 9),确定分子式为C36H34N2O6,结合该化合物的13C NMR和DEPT的数据进一步确定不饱和度为21(表1)。然而,从1H NMR谱和13C NMR谱只观察到17个氢和18个碳的信号,这暗示了化合物1的结构具有对称性。结合上述分析,根据1H NMR谱和13C NMR谱中所有信号可归属给2个非共轭羰基、2个亚甲二氧苯基单元、2个苯环单元、4个亚甲基和4个次甲基(表1)。
-
通过1H-1H COSY谱中的信号表明,-NH/H-10/H-11存在相关,H-13/H-14/H-15存在相关。同时,在HMBC谱中可以观察到以下关系:H-10与C-9、C-11和C-12存在相关;H-2与C-3、C-6和C-7存在相关;H-6与C-1、C-2、C-4、C-5和C-7存在相关;H-18与C-3和C-4存在相关;H-7与C-1、C-2、C-6、C-8和C-9存在相关;H-11与C-10、C-12和C-13存在相关。以上1H-1H COSY和HMBC数据并结合文献推测(Wei et al.,2005),分子结构中存在1个亚甲二氧苯基和1个9-氧-10,11-亚甲基苯结构片段,它们之间通过一个由2个次甲基组成的二碳单位连接在一起,这些信息给出了化合物分子一半的结构单元。质谱片段在m/z 296.128 1 [C18H17N1O3+H]+处显示出特征信号,也证实了上述结论。通过质子H-6与H-7和H-8的NOESY相关性以及NH与H-7和H-8的NOESY相关性并结合文献(Zhou et al.,2017)表明H-7和H-8与H-7′和H-8′之间的关系为顺式构型,而H-7和H-7′与H-8和H-8′之间的关系为反式构型,这表明化合物1具有头到头构型的环丁烷环(—H-7—H-8—H-8′—H-7′—),并且1个亚甲二氧苯基和1个9-氧-10,11-亚甲基苯结构片段是同向的。因此,可以确定化合物1的结构(图1)。经Scifinder数据库检索,化合物1为一个未见文献报道的新化合物,其氢谱和碳谱数据见表1。因此,清楚地确定了化合物1的结构,并将化合物1命名为pipercyclobutanamide C。
-
其他化合物结构式见图2。
-
图1 化合物1的HMBC(→)、1H-1H COSY(—) 和NOSEY(
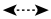 )的关键相关
)的关键相关 -
Fig.1 Key HMBC (→) , 1H-1H COSY (—) and NOSEY (
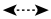 ) correlations signal of Compound 1
) correlations signal of Compound 1 -
化合物2 白色粉末,分子式为C36H40O9;1H NMR(600 MHz,CD3OD)δ: 0.67(1H,m,H-2′α),0.70(1H,m,H-2β),0.97(3H,s,H-14′),1.21(3H,s,H-14),1.26(1H,m,H-2α),1.28(1H,m,H-2′β),1.39(3H,s,H-13),1.75(1H,m,H-1′),1.78(1H,m,H-3′),1.78(1H,m,H-5′),1.79(1H,m,H-6′β),1.87(1H,m,H-4″),1.88(1H,m,H-5″),1.90(1H,m,H-3),2.10(1H,m,H-1),2.20(1H,m,H-15α),2.50(1H,m,H-6′α),2.63(3H,dd,J = 9.0,7.2 Hz,H-9′),3.12(1H,dd,J = 18.6,7.2 Hz,H-15β),3.47(3H,s,MeO-12),3.95(1H,s,H-9),4.16(1H,d,J = 10.8 Hz,H-15′α),4.25(1H,d,J = 10.8 Hz,H-15′β),5.64(1H,s,H-13′α),6.21(1H,s,H-13′β),6.94(1H,m,H-3″); 13C NMR(150 MHz,CD3OD)δ: 28.6(C-1),15.2(C-2),27.6(C-3),151.4(C-4),136.4(C-5),152.9(C-6),129.6(C-7),198.6(C-8),84.6(C-9),58.4(C-10),66.6(C-11),173.7(C-12),19.0(C-13),15.2(C-14),30.1(C-15),27.3(C-1′),10.6(C-2′),30.4(C-3′),79.3(C-4′),55.1(C-5′),29.1(C-6′),60.5(C-7′),97.6(C-8′),53.8(C-9′),43.9(C-10′),148.1(C-11′),168.8(C-12′),123.4(C-13′),24.3(C-14′),68.5(C-15′),170.3(C-1″),131.8(C-2″),139.2(C-3″),14.6(C-4″),12.4(C-5″),52.3(MeO-12)。以上理化性质和波谱数据与文献(Shen et al.,2017)报道的chololactone A基本一致,最终判断化合物2为chololactone A。
-
化合物3 白色粉末,分子式为C36H40O10;1H NMR(600 MHz,CDCl3)δ: 0.67(1H,m,H-2′α),0.92(3H,s,H-14′),0.96(1H,m,H-2α),1.10(3H,s,H-14),1.20(1H,m,H-2′β),1.22(1H,m,H-2β),1.32(3H,s,H-13),1.64(1H,m,H-15′),1.77(1H,m,H-3′),1.79(1H,m,H-6′β),1.81(3H,m,H-4″),1.82(1H,m,H-1′),1.84(3H,m,H-5″),1.87(1H,m,H-6′α),2.12(1H,dd,J = 8.4,3.6 Hz,H-3),2.44(1H,s,OH-5),3.13(1H,s,H-9′),3.41(1H,s,OH-9),3.66(3H,s,J = 12.0 Hz,MeO-12),3.89(1H,d,J = 11.4 Hz,H-15′β),4.03(1H,m,H-15′α),4.05(1H,m,H-9),5.74(1H,s,H-13′α),6.14(1H,s,H-15α),6.55(1H,s,H-13′β),6.86(1H,d,J = 7.2 Hz,H-3″); 13C NMR(150 MHz,CDCl3)δ: 28.4(C-1),12.3(C-2),22.1(C-3),148.9(C-4),72.1(C-5),150.7(C-6),137.2(C-7),198.3(C-8),80.9(C-9),56.6(C-10),63.8(C-11),172.6(C-12),25.5(C-13),11.8(C-14),118.3(C-15),28.2(C-1′),10.5(C-2′),29.3(C-3′),78.6(C-4′),53.4(C-5′),32.5(C-6′),57.9(C-7′),95.1(C-8′),54.4(C-9′),42.8(C-10′),143.7(C-11′),167.7(C-12′),125.9(C-13′),23.1(C-14′),68.7(C-15′),167.8(C-1″),128.2(C-2″),138.2(C-3″),14.5(C-4″),12.1(C-5″),52.8(MeO-12)。以上理化性质和波谱数据与文献(He et al.,2011)报道的sarcanolide B基本一致,最终判断化合物3为sarcanolide B。
-
化合物4 无定形粉末,分子式为C32H36O8;1H NMR(600 MHz,CDCl3)δ: 0.22(2H,m,H-2β,H-2′β),0.87(2H,m,H-2α,H-2′α),1.00(6H,s,H-14,H-14′),1.51(6H,s,H-13,H-13′),1.84(2H,m,H-1,H-1′),1.98(2H,s,H-3,H-3′),2.60(2H,d,J = 13.2 Hz,H-15β,H-15′β),2.96(2H,d,J=13.2 Hz,H-15α,H-15′α),3.65(6H,s,MeO-12),4.01(2H,s,H-9,H-9′),7.16(2H,s,H-6,H-6′); 13C NMR(150 MHz,CDCl3)δ: 24.7(C-1,C-1′),14.0(C-2,C-2′),27.9(C-3,C-3′),147.4(C-4,C-4′),135.8(C-5,C-5′),138.4(C-6,C-6′),137.3(C-7,C-7′),199.0(C-8,C-8′),81.0(C-9,C-9′),58.5(C-10,C-10′),47.8(C-11,C-11′),175.5(C-12,C-12′),28.5(C-13,C-13′),15.9(C-14,C-14′),38.3(C-15,C-15′),52.1(MeO-12,12′)。以上理化性质和波谱数据与文献(Kwon et al.,2006)报道的环银线草醇A基本一致,最终判断化合物4为环银线草醇A。
-
化合物5 无色无定形粉末,分子式为C35H38O11;1H NMR(600 MHz,CDCl3)δ: 0.63(1H,m,H-2′α),0.95(1H,m,H-2α),1.03(3H,s,H-14′),1.13(1H,m,H-2β),1.16(3H,s,H-14),1.21(1H,m,H-2′β),1.54(1H,m,H-1′),1.63(3H,m,H-13),1.81(3H,m,H-5″),1.82(1H,m,H-3),1.86(1H,s,H-15α),2.19(1H,m,H-1),2.35(1H,m,H-5′),2.45(1H,m,H-6′α),2.63(1H,m,H-9′),2.71(1H,m,H-1′),2.92(1H,dd,J = 17.4,12.6 Hz,H-6′β),4.05(1H,d,J = 11.4 Hz,H-15′β),4.08(1H,d,J = 11.4 Hz,H-15′α),4.42(1H,dd,J = 13.2,10.8 Hz,H-13′β),4.48(1H,dd,J = 13.2,5.4 Hz,H-13′α),6.86(1H,m,H-3″); 13C NMR(150 MHz,CDCl3)δ: 24.2(C-1),9.1(C-2),30.4(C-3),77.1(C-4),160.4(C-5),123.2(C-6),150.4(C-7),95.1(C-8),199.9(C-9),56.8(C-10),127.3(C-11),171.4(C-12),10.9(C-13),21.1(C-14),40.1(C-15),26.5(C-1′),10.3(C-2′),29.6(C-3′),77.1(C-4′),52.9(C-5′),21.6(C-6′),168.1(C-7′),85.6(C-8′),50.9(C-9′),44.5(C-10′),127.3(C-11′),172.5(C-12′),54.3(C-13′),24.0(C-14′),71.1(C-15′),169.0(C-1″),128.0(C-2″),139.0(C-3″),14.3(C-4″),11.8(C-5″)。以上理化性质和波谱数据与文献(Xu et al.,2007)报道的chloramultiol D基本一致,最终判断化合物5为chloramultiol D。
-
化合物6 无色无定形粉末,分子式为C32H33O5;1H NMR(600 MHz,CDCl3)δ: 0.66(1H,m,H-2′),0.75(1H,m,H-2′),0.88(3H,s,H-14′),1.02(1H,m,H-2),1.14(3H,s,H-14),1.22(1H,m,H-3′),1.23(1H,m,H-2),1.41(1H,m,H-1′),1.62(1H,m,H-4′),1.71(3H,s,H-13),1.81(1H,m,H-3),1.83(1H,m,H-15),1.83(1H,m,H-3),2.07(3H,s,H-2″),2.17(1H,m,H-5′),2.25(1H,m,H-1α),2.29(1H,m,H-1β),2.56(1H,m,H-6′),2.63(1H,m,H-6′),2.70(1H,m,H-15),2.71(1H,dd,J=15.6,6.6 Hz,H-9′),3.90(1H,m,H-15′β),4.04(1H,dd,J = 10.8,4.8 Hz,H-15′α),4.41(1H,d,J = 14.4 Hz,H-13′β),4.48(1H,d,J = 14.4 Hz,H-13′α); 13C NMR(150 MHz,CDCl3)δ: 24.2(C-1),9.3(C-2),30.1(C-3),77.3(C-4),160.4(C-5),123.2(C-6),149.8(C-7),95.0(C-8),199.4(C-9),57.0(C-10),127.0(C-11),171.2(C-12),11.2(C-13),21.1(C-14),39.7(C-15),25.8(C-1′),16.2(C-2′),23.4(C-3′),44.7(C-4′),51.6(C-5′),25.1(C-6′),166.7(C-7′),85.4(C-8′),50.4(C-9′),43.9(C-10′),127.0(C-11′),171.5(C-12′),55.7(C-13′),22.4(C-14′),67.7(C-15′),172.8(C-1″),21.2(C-2″)。以上理化性质和波谱数据与文献(Wang et al.,2015)报道的chlorasessilifol B基本一致,最终判断化合物6为chlorasessilifol B。
-
化合物7 白色粉末,分子式C41H48O13;1H NMR(600 MHz,CD3OD)δ: 0.34(1H,dd,J=7.8,4.2 Hz,H-2β),0.75(1H,m,H-2′β),0.91(3H,s,H-14′),0.99(1H,m,H-2α),1.06(3H,s,H-14),1.31(1H,dd,J=9.6,4.2 Hz,H-2′α),1.47(1H,m,H-3′),1.69(1H,m,H-1′),1.77(1H,m,H-1),1.80(1H,dd,J=6.0 Hz,H-9′),1.83(3H,s,H-13),1.85(1H,m,H-5″),1.88(1H,m,H-4″),1.95(1H,m,H-3),2.45(1H,dd,J=18.6,6.0 Hz,H-6β),2.55(1H,ddd,J=10.2,6.0,4.8 Hz,H-15β),2.82(1H,m,H-6α),2.86(1H,m,H-15α),3.32(3H,s,MeO-1’’’),3.70(3H,s,MeO-12),3.79(1H,d,J=11.4 Hz,H-15′β),4.76(1H,d,J=13.2 Hz,H-13′β),4.87(1H,d,J=9.0 Hz,H-13′α),3.90(1H,s,H-9),3.98(1H,d,J=3.0 Hz,H-6),4.23(1H,d,J=11.4 Hz,H-15′α),6.96(1H,m,H-3″); 13C NMR(150 MHz,CD3OD)δ: 26.7(C-1),16.1(C-2),25.4(C-3),143.1(C-4),133.7(C-5),41.9(C-6),134.7(C-7),202.1(C-8),80.9(C-9),52.4(C-10),146.6(C-11),172.1(C-12),20.1(C-13),15.8(C-14),26.0(C-15),26.4(C-1′),12.5(C-2′),29.1(C-3′),77.7(C-4′),62.4(C-5′),24.2(C-6′),17.5(C-7′),94.8(C-8′),57.3(C-9′),46.1(C-10′),124.6(C-11′),173.4(C-12′),55.9(C-13′),26.8(C-14′),72.8(C-15′),170.1(C-1″),129.5(C-2″),139.5(C-3″),14.6(C-4″),12.4(C-5″),173.7(C-1’’’),29.1(C-2’’’),29.1(C-3’’’),174.5(C-4’’’),52.9(MeO-12),52.3(MeO-1’’’)。以上理化性质和波谱数据与文献(Wang et al.,2011)报道的chlorajaponal基本一致,最终判断化合物7为chlorajaponal。
-
化合物8 白色粉末,分子式为C39H42O14;1H NMR(600 MHz,CDCl3)δ: 0.60(1H,dt,J = 12.0 Hz,H-2′β),0.80(1H,m,H-2α),0.83(3H,s,H-14),0.93(3H,s,H-14′),1.04(1H,dd,J = 12.0,6.0 Hz,H-2β),1.22(1H,d,J = 6.0 Hz,H-2′α),1.46(1H,m,H-3′),1.62(3H,s,H-13),1.65(1H,m,H-1′),1.80(1H,m,H-3),1.80(1H,m,H-15β),1.83(1H,m,H-1),1.85(1H,dd,H-5″),2.36(1H,dd,J = 12.0 Hz,H-5′),2.53(1H,m,H-2’’’β),2.60(1H,m,H-3’’’β),2.64(1H,m,H-9′),2.66(1H,m,H-2’’’α),2.66(1H,m,H-3’’’α),2.70(1H,m,H-6′β),2.72(1H,m,H-15α),2.97(1H,dd,J = 18.0,12.0 Hz,H-6′α),3.75(1H,s,H-9),3.87(1H,d,J = 12.0 Hz,H-15′β),4.51(1H,d,J = 12.0 Hz,H-13′β),4.59(1H,d,J = 12.0 Hz,H-15′α),4.64(1H,dd,J = 12.0,6.0 Hz,H-4″β),4.84(1H,dd,J = 12.0,6.0 Hz,H-4″α),5.17(1H,d,J = 12.0 Hz,H-13′α),6.72(1H,m,H-3″); 13C NMR(150 MHz,CDCl3)δ: 29.9(C-1),9.7(C-2),31.5(C-3),78.2(C-4),164.6(C-5),124.3(C-6),154.5(C-7),105.0(C-8),80.0(C-9),51.1(C-10),125.4(C-11),174.6(C-12),10.8(C-13),14.4(C-14),41.5(C-15),27.8(C-1′),10.8(C-2′),30.3(C-3′),78.2(C-4′),56.2(C-5′),25.0(C-6′),177.4(C-7′),87.4(C-8′),52.0(C-9′),46.6(C-10′),124.0(C-11′),173.4(C-12′),51.0(C-13′),24.4(C-14′),74.8(C-15′),168.9(C-1″),130.3(C-2″),137.7(C-3″),62.8(C-4″),12.8(C-5″),174.2(C-1’’’),29.8(C-2’’’),29.8(C-3’’’),173.9(C-4’’’)。以上理化性质和波谱数据与文献(Kim et al.,2009)报道的tianmushanol基本一致,最终判断化合物8为tianmushanol。
-
化合物9 白色粉末,分子式为C40H44O14;1H NMR(600 MHz,CDCl3)δ: 0.65(1H,m,H-2′),0.82(3H,s,H-14),0.92(3H,s,H-14′),0.95(1H,m,H-2),1.16(1H,m,H-2),1.29(1H,m,H-2′),1.46(1H,m,H-3′),1.59(1H,m,H-1′),1.69(1H,m,H-15),1.71(3H,s,H-13),1.82(1H,m,H-3),1.93(3H,s,H-e),1.94(1H,m,H-1),2.14(1H,dd,J = 12.0,7.1 Hz,H-5′),2.47(1H,m,H-g),2.49(1H,m,H-15),2.54(1H,m,H-h),2.54(1H,m,H-6′),2.62(1H,s,H-9′),2.66(1H,m,H-h),2.68(1H,m,H-g),2.92(1H,m,H-6′),3.44(3H,s,-OCH3),3.73(1H,d,J = 12.0 Hz,H-15′),3.83(3H,s,H-9),4.52(1H,d,J = 12.0 Hz,H-13′),4.66(1H,dd,J = 12.0 Hz,H-d),4.73(1H,dd,J = 12.0 Hz,H-d),4.92(1H,d,J = 12.0 Hz,H-15′),5.23(1H,d,J = 12.0 Hz,H-13′),6.60(1H,m,H-c); 13C NMR(150 MHz,CDCl3)δ: 29.2(C-1),9.6(C-2),29.7(C-3),77.5(C-4),164.3(C-5),122.4(C-6),150.8(C-7),105.0(C-8),75.5(C-9),49.7(C-10),125.7(C-11),170.8(C-12),10.7(C-13),14.0(C-14),40.2(C-15),26.4(C-1′),10.3(C-2′),29.0(C-3′),77.5(C-4′),55.8(C-5′),23.5(C-6′),174.0(C-7′),85.3(C-8′),51.1(C-9′),45.0(C-10′),123.3(C-11′),171.3(C-12′),53.7(C-13′),24.1(C-14′),72.8(C-15′),167.6(C-a),130.6(C-b),135.6(C-c),60.8(C-d),12.8(C-e),171.8(C-f),28.8(C-g),29.0(C-h),171.8(C-i),52.2(MeO-8)。以上理化性质和波谱数据与文献(Ran et al.,2010)报道的spicachlorantins B基本一致,最终判断化合物9为spicachlorantins B。
-
化合物10 白色粉末,分子式为C39H40O14;1H NMR(600 MHz,CDCl3)δ: 0.64(1H,dt,J = 12.0,6.0 Hz,H-2′β),0.96(3H,s,H-14′),1.03(1H,m,H-2β),1.16(3H,s,H-14),1.23(1H,m,H-2α),1.28(1H,m,H-2′α),1.46(1H,m,H-3′),1.56(1H,m,H-1′),1.74(1H,m,H-15β),1.80(1H,m,H-3),1.82(3H,s,H-13),1.86(3H,m,H-5″),2.22(1H,dd,J = 12.0,6.0 Hz,H-5′),2.29(1H,m,H-1),2.40(1H,dd,J = 18.0,6.0 Hz,H-6′β),2.50(1H,m,H-3’’’β),2.53(1H,m,H-2’’’β),2.59(1H,dd,J = 12.0,6.0 Hz,H-9′),2.67(1H,m,H-15α),2.73(1H,m,H-3’’’α),2.77(1H,m,H-2’’’α),3.04(1H,dd,J = 18.0 Hz,H-6′α),4.03(1H,d,J = 12.0 Hz,H-15′β),4.33(1H,d,J = 12.0 Hz,H-15′α),4.51(1H,d,J = 12.0 Hz,H-13′β),4.63(1H,dd,J = 12.0,6.0 Hz,H-4″β),4.75(1H,dd,J = 12.0 Hz,H-4″α),5.40(1H,d,J = 12.0 Hz,H-13′α),6.57(1H,dd,J = 6.0 Hz,H-3″); 13C NMR(150 MHz,CDCl3)δ: 24.2(C-1),9.3(C-2),30.1(C-3),77.5(C-4),160.7(C-5),123.7(C-6),136.4(C-7),94.0(C-8),200.1(C-9),56.9(C-10),129.4(C-11),170.2(C-12),11.3(C-13),20.8(C-14),40.0(C-15),26.7(C-1′),9.9(C-2′),29.7(C-3′),77.5(C-4′),54.9(C-5′),24.0(C-6′),173.5(C-7′),85.5(C-8′),51.9(C-9′),45.1(C-10′),123.7(C-11′),170.7(C-12′),53.3(C-13′),24.2(C-14′),74.1(C-15′),167.8(C-1″),129.4(C-2″),136.4(C-3″),61.7(C-4″),12.8(C-5″),172.1(C-1’’’),28.8(C-2’’’),28.8(C-3’’’),172.1(C-4’’’)。以上理化性质和波谱数据与文献(Wu et al.,2008)报道的spicachlorantins A基本一致,最终判断化合物10为spicachlorantins A。
-
化合物11 白色粉末,分子式为C41H44O14;1H NMR(600 MHz,CDCl3)δ: 0.67(1H,ddd,J = 9.2,8.5,5.2 Hz,H-2′α),0.98(1H,m,H-2),1.00(3H,s,H-14′),1.12(3H,t,J = 7.2 Hz,-CH3),1.20(3H,s,H-14),1.29(1H,m,H-2),1.30(1H,m,H-2′β),1.53(1H,ddd,J = 9.2,7.5,3.5 Hz,H-3′),1.74(1H,ddd,J = 9.2,8.5,4.6 Hz,H-1′),1.79(3H,s,H-13),1.88(3H,s,H-e),1.90(1H,m,H-15),1.93(1H,m,H-3),2.07(1H,ddd,J = 7.9,6.5,4.0 Hz,H-1),2.08(1H,m,H-6′),2.53(1H,dd,J = 17.8,7.5 Hz,H-5′),2.57(1H,m,H-h),2.58(1H,m,H-g),2.62(1H,m,H-h),2.64(1H,m,H-g),2.73(1H,m,H-15),2.75(1H,s,H-9′),3.10(1H,m,H-6′),3.74(1H,m,-OCH2-),3.97(1H,t,J = 6.0 Hz,H-c),4.02(1H,m,-OCH2-),4.58(1H,d,J = 12.0 Hz,H-15′),4.60(1H,d,J = 12.3 Hz,H-13′),4.69(1H,m,H-d),4.90(3H,s,H-e),5.30(1H,d,J = 12.3 Hz,H-13′),6.68(1H,t,J = 6.0 Hz,H-c); 13C NMR(150 MHz,CDCl3)δ: 26.3(C-1),10.9(C-2),32.1(C-3),77.8(C-4),162.5(C-5),118.5(C-6),151.9(C-7),101.5(C-8),205.1(C-9),56.6(C-10),129.4(C-11),172.1(C-12),12.2(C-13),24.0(C-14),41.1(C-15),27.9(C-1′),11.4(C-2′),30.1(C-3′),77.6(C-4′),56.1(C-5′),25.7(C-6′),176.8(C-7′),87.8(C-8′),52.8(C-9′),48.7(C-10′),125.4(C-11′),173.5(C-12′),55.8(C-13′),24.6(C-14′),75.2(C-15′),168.3(C-a),130.7(C-b),137.9(C-c),62.6(C-d),12.8(C-e),173.5(C-f),30.0(C-g),30.0(C-h),173.4(C-i),64.0(-OCH2-),16.6(MeO-12)。以上理化性质和波谱数据与文献(Zhang et al.,2012)报道的及已灵素A基本一致,最终判断化合物11为及已灵素A。
-
化合物12 白色粉末,分子式为C40H44O14;1H NMR(600 MHz,CDCl3)δ: 0.41(1H,m,H-2α),0.82(1H,m,H-2′β),0.84(3H,s,H-14′),1.03(3H,s,H-14),1.04(1H,m,H-2β),1.29(1H,m,H-2′α),1.31(1H,m,H-3′),1.43(1H,m,H-1′),1.71(1H,m,H-5′),1.87(1H,m,H-3),1.88(3H,s,H-5″),1.88(3H,s,H-13),1.99(1H,d,J= 2.2 Hz,H-9′),2.04(1H,m,H-1),2.39(1H,m,H-6″β),2.43(1H,m,H-8″α),2.71(1H,m,H-7″α),2.74(1H,m,H-7″β),2.74(1H,m,H-6′α),2.85(1H,m,H-8″β),3.57(1H,d,J = 11.8 Hz,H-15′β),3.64(3H,s,MeO-12),3.81(1H,s,H-9),4.09(1H,s,H-6),4.47(1H,d,J = 11.8 Hz,H-15′α),4.49(1H,dd,J = 14.7,4.5 Hz,H-4″α),4.49(1H,d,J = 11.9 Hz,H-13′β),4.57(1H,dd,J = 15.0,4.6 Hz,H-4″β),4.85(3H,br s,H-15),5.04(1H,d,J = 11.8 Hz,H-13′α),6.55(1H,m,H-3″); 13C NMR(150 MHz,CDCl3)δ: 25.5(C-1),16.4(C-2),23.5(C-3),144.9(C-4),136.6(C-5),41.0(C-6),132.1(C-7),200.3(C-8),79.8(C-9),51.4(C-10),147.9(C-11),169.9(C-12),20.1(C-13),15.0(C-14),65.1(C-15),25.1(C-1′),11.6(C-2′),27.8(C-3′),76.6(C-4′),59.2(C-5′),23.1(C-6′),173.4(C-7′),91.9(C-8′),62.2(C-9′),43.9(C-10′),123.7(C-11′),172.0(C-12′),54.2(C-13′),26.2(C-14′),72.0(C-15′),167.0(C-1″),129.1(C-2″),135.5(C-3″),61.6(C-4″),13.0(C-5″),171.9(C-6″),28.6(C-7″),29.1(C-8″),172.0(C-9″),52.4(MeO-12)。以上理化性质和波谱数据与文献(Ran et al.,2010)报道的chloramultiols A基本一致,最终判断化合物12 为chloramultiols A。
-
图2 化合物1-12的化学结构
-
Fig.2 Chemical structures of compounds 1-12
-
4 药理活性研究
-
采用MTT法初步研究化合物1-12对人宫颈癌细胞HeLa、人乳腺癌细胞MCF-7、人前列腺癌细胞DU-145、人肺癌细胞A549和人脑胶质瘤细胞T98G的细胞毒活性,以紫杉醇为阳性对照组。细胞毒活性测试结果显示,仅化合物2和7对人宫颈癌细胞HeLa有抑制作用,其他化合物均无抑制作用(表2)。
-
5 讨论与结论
-
本研究通过用硅胶柱层析、反相柱色谱、Sephadex LH-20凝胶和半制备高效液相等色谱技术对宽叶金粟兰95%乙醇提取物的乙酸乙酯部位分离并鉴定,得到12个化合物,包括一个新的环丁烷酰胺类化合物和11个倍半萜二聚体类化合物。其中,化合物1为新化合物,化合物2-7、9-12首次从宽叶金粟兰中分离得到。通过MTT法测试化合物细胞毒活性,结果表明仅化合物2和7对人宫颈癌细胞HeLa有抑制作用,IC50分别为(4.50±0.27) μmol·L-1和(4.25±0.08) μmol·L-1,其他化合物均无抑制作用。此外,我们对宽叶金粟兰中分离的化合物进行了体外抗HIV-1细胞活性筛选,通过对HIV-1的感染性滴定、C8166细胞毒性和HIV-1ⅢB致细胞病变(CPE)的抑制实验,结果表明化合物7对C8166细胞的毒性极大,CC50低于1 μmol·L-1。所有化合物对HIV-1ⅢB引起的合胞体形成的抑制作用弱,TI值均低于2,均无明显的抗HIV-1细胞活性。
-
有研究发现,从金粟兰植物中分离得到倍半萜二聚体化合物,并采用SYBR-Green抗恶性疟原虫Dd2(耐氯喹)检测法检测所有化合物的抗疟原虫活性(Guo et al.,2016;Zhou et al.,2017)。其中,化合物fortunilide A、sarglabolide J和chlorajaponilide C的IC50值分别为(5.2±0.6) nmol·L-1、(7.2±1.3) nmol·L-1 和(1.1±0.2) nmol·L-1,与阳性对照青蒿素[IC50=(4.0±4.2)nmol·L-1]具有相似的效力。从金粟兰属植物银线草(C. japonicus)分离得到的倍半萜二聚体化合物chlojapolide A、shizukaol B、shizukaol G和shizukaol D对RAW 264.7巨噬细胞脂多糖(LPS)诱导的一氧化氮(NO)产生表现出明显的抑制作用,IC50值在6.91~15.75 μmol·L-1范围内,比阳性对照槲皮素(IC50=15.90 μmol·L-1)更活跃。因此,在后续研究中将评价倍半萜二聚体化合物的抗疟原虫和抗炎活性,对含量较大的化合物7进行部分结构修饰并进行抗疟原虫和抗炎活性检测,进一步探索倍半萜二聚体成分的构效关系与作用机制,以期寻找到更多具有生物活性的天然产物,同时也将开展更广泛的其他生物活性评价,为宽叶金粟兰的开发利用奠定基础。
-
参考文献
-
CAO CM, PENG Y, XIAO PG, et al. , 2008. Advance in on chemical constituent and bioactivity research of genus Chloranthus [J]. China J Chin Mat Med, (13): 1509-1515. [曹聪梅, 彭勇, 肖培根. 金粟兰属植物的化学成分和药理作用研究进展 [J]. 中国中药杂志, (13): 1509-1515. ]
-
GUO YQ, ZHAO JJ, LI ZZ, et al. , 2016. Natural nitric oxide (NO) inhibitors from Chloranthus japonicus [J]. Bioorg Med Chem Lett, 26(13): 3163-3166.
-
HE XF, ZHANG S, ZHU RX, et al. , 2011. Sarcanolides A and B: two sesquiterpenoid dimers with a nonacyclic scaffold from Sarcandra hainanensis [J]. Tetrahedron, 67(18): 3170-3174.
-
KIM SY, KASHIWADA Y, KAWAZOE K, et al. , 2009. Spicachlorantins A and B, new dimeric sesquiterpenes from the roots of Chloranthus spicatus [J]. Phytochem Lett, 2(3): 110-113.
-
KWON OE, LEE HS, LEE SW, et al. , 2006. Dimeric sesquiterpenoids isolated from Chloranthus japonicus inhibited the expression of cell adhesion molecules [J]. J Ethnopharmacol, 104(1/2): 270-277.
-
LUO G, MA XX, LUO YM, et al. , 2014. Studies on the chemical constituents from the roots of Chloranthus henryi [J]. China J Exp Tradit Med Form, 20(1): 47-50. [罗杠, 马兴霞, 罗永明, 等, 2014. HPLC同时测定金粟兰植物中香豆素及倍半萜内酯类有效成分的含量 [J]. 中国实验方剂学杂志, 20 (1): 47-50. ]
-
RAN XH, TENG F, CHEN CX, et al. , 2010. Chloramultiols A-F, lindenane-type sesquiterpenoid dimers from Chloranthus multistachys [J]. J Nat Prod, 73(5): 972-975.
-
SHEN CP, LUO JG, YANG MH, et al. , 2017. Sesquiterpene dimers from the roots of Chloranthus holostegius with moderate anti-inflammatory activity [J]. Phytochemistry, 137: 117-122.
-
WANG LJ, XIONG J, LAU C, et al. , 2015. Sesquiterpenoids and further diterpenoids from the rare Chloranthaceae plant Chloranthus sessilifolius [J]. J Asian Nat Prod Res, 17(12): 1220-1230.
-
WANG QH, KUANG HX, YANG BY, et al. , 2011. Sesquiterpenes from Chloranthus japonicus [J]. J Nat Prod, 74(1): 16-20.
-
WEI K, LI W, KOIKE K, et al. , 2005. Nigramides A-S, dimeric amide alkaloids from the roots of Piper nigrum [J]. J Org Chem, 70(4): 1164-1176.
-
WU B, CHEN J, QU H, et al. , 2008. Complex sesquiterpenoids with tyrosinase inhibitory activity from the leaves of Chloranthus tianmushanensis [J]. J Nat Prod, 71(5): 877-880.
-
XU YJ, TANG CP, KE CQ, et al. , 2007. Mono- and di-sesquiterpenoids from Chloranthus spicatus [J]. J Nat Prod, 70(12): 1987-1990.
-
YANG XW, 2017. Bioactive material basis of medicinal plants in genus Chloranthus [J]. Mod Chin Med, 19(4): 459-481. [杨秀伟, 2017. 金粟兰属药用植物的物质基础 [J]. 中国现代中药, 19(4): 459-481. ]
-
ZHANG M, IINUMA M, WANG JS, et al. , 2012. Terpenoids from Chloranthus serratus and their anti-inflammatory activities [J]. J Nat Prod, 75(4): 694-698.
-
ZHANG SS, FU JJ, CHEN HY, et al. , 2017. Sesquiterpenes with anti-metastasis breast cancer activity from Chloranthus henryi [J]. China J Chin Mat Med, 42(20): 3938-3944. [张珊珊, 付剑江, 陈怀远, 等, 2017. 宽叶金粟兰中具抗乳腺癌转移活性的倍半萜类化学成分研究 [J]. 中国中药杂志, 42(20): 3938-3944. ]
-
ZHOU B, WU Y, DALAL S, et al. , 2017. Nanomolar antimalarial agents against chloroquine-resistant Plasmodium falciparum from medicinal plants and their structure-activity relationships [J]. J Nat Prod, 80(1): 96-107.
-
摘要
为探寻宽叶金粟兰(Chloranthus henryi)的化学成分及抗肿瘤活性,该研究采用硅胶柱层析、反相柱色谱、Sephadex LH-20凝胶和半制备高效液相等色谱技术对宽叶金粟兰95%乙醇提取物的乙酸乙酯部位进行分离纯化,根据理化性质与波谱数据并结合参考文献鉴定所得化合物的结构,并采用MTT法评价化合物细胞毒活性。结果表明:(1)从该植物的95%乙醇提取物的乙酸乙酯部位分离并鉴定了12个化合物,分别为pipercyclobutanamide C(1)、chololactone A(2)、sarcanolide B(3)、环银线草醇A(4)、chloramultiol D(5)、chlorasessilifol B(6)、chlorajaponol(7)、tianmushanol(8)、spicachlorantins B(9)、spicachlorantins A(10)、及已灵素A(11)、chloramultiols A(12)。化合物1为新化合物,除化合物8外,其余化合物均为首次从宽叶金粟兰中分离得到。(2)细胞毒活性测试结果显示,仅化合物2和化合物7对人宫颈癌细胞HeLa有抑制作用,IC50值分别为(4.50±0.27) μmol·L-1和(4.25±0.08) μmol·L-1,其他化合物对其均无抑制作用。该研究结果为进一步深度研究与开发利用金粟兰属草本植物提供了一定的科学依据。
Abstract
In order to study the chemical constituents from Chloranthus henryi and their antitumor activities in vitro. The chemical constituents of ethyl acetate fraction, extracted with 95% ethanol from C. henryi were isolated and purified by silica gel column chromatography, reversed phase column chromatography, Sephadex LH-20 column chromatography and preparation liquid chromatography, and their structures were identified by the physicochemical properties, spectral data combined with relevant literatures. The cytotoxic activities of these compounds were evaluated by MTT method. The results were as follows: (1) Twelve compounds were isolated and identified as pipercyclobutanamide C (1), chololactone A (2), sarcanolide B (3), oxacol A (4), chloramultiol D (5), chlorasessilifol B (6), chlorajaponol (7), tianmushanol (8), spicachlorantins B (9), spicachlorantins A (10), serrachlorin A (11), chloramultiols A (12). Among them, Compound 1 was identified as a new compound, and except for Compound 8, all the other compounds were isolated from the C. henryi for the first time. (2) The cytotoxic activity test results showed that only compounds 2 and 7 had good inhibitory effects on HeLa cells in vitro, while the other compounds showed no inhibition effects. The IC50 of the two compounds were (4.50±0.27) μmol·L-1 and (4.25±0.08) μmol·L-1 respectively. In conclusion, the study enriches the chemical constituents of C. henryi, and provides a reference for the further exploration and utilization of this Chloranthus herb plants.


